Submitting an ethics application is a daunting process. There are so many things to consider, and this is multiplied when working with a participant population which may be vulnerable. So, what do you need to consider when applying for ethical approval for your research? I am going to talk you through some points you need to consider!
Having just submitted my own ethics application for focus groups and an online survey, I feel I can now save you some time and talk about the sources I have found helpful and what needs to be included for you to (hopefully!) get the green light to carry out your research.
I first need to specify that the advice here will be for carrying out research with people living with dementia who have the capacity to consent. If you are planning research with people who may be unable to give written or verbal consent or will be going through NHS ethics, I will provide some signposting to research and support, but I will not be able to advise much further sadly. This blog will also be most relevant to those conducting qualitative research. I will begin with what you need to consider generally before going on to considerations that need to be made specifically for research with people living with dementia.
What to do before you even begin
- Find out when the ethics board for your organisation/university meets – this may be monthly or less often so you may need to plan for the deadline.
- Find out if there are templates for forms such as the Participant Information Sheet, Risk Assessment etc. – this will save you lots of time and prevent needless mistakes!
- Find out where the ethics submission needs to be sent (i.e., is there a specific person you need to email or online portal for doing this?)
- If you have questions about the ethics submission there will be a person who you can contact with questions about the submission process, they can be really useful!
- Speak with your supervisors/colleagues about the plans for the research you are submitting and ask them questions if you are not sure – this will save you time as you do not want your ethics submission rejected over a small error that could have been amended by asking someone.
- Find out what the procedure is for if your ethics submission is not approved the first time (i.e., if it requires amendments, how long will it take the board to re-evaluate your ethics submission?) – this may set you back or may be a quick turn around so is worth finding out so you can factor this into your plans.
Forms, forms, and more forms!
Now that you’ve found out all the admin details you will need, it’s time to fill out many, many forms! It is worth considering that if you are applying for ethics for multiple elements, e.g., an online survey and focus groups, many of the forms will have to be submitted for each part of the proposed research. For example, Online Survey Risk Assessment and Focus Group Risk Assessment.
The main forms you can expect to submit are:
- Participant information sheet
- Informed consent form
- Risk assessment
- In the case of conducting surveys, interviews or focus groups you will need to submit sample questions/topic guides – enough detail that the ethics board gets an idea of what you plan to do and shows you have thought through what you would do in practice
- It is likely you will also need to submit a document with further details of the research (aims and objectives, non-specialist summary of study background, study method, investigator experience and risks to participants).
What you need for each form
I will now talk through the details you are likely to need to include for each form/document. Templates will likely be available, which you can adapt to your own research, which makes the process quicker. You will however need to think about any additional points that you want to include on these generic forms.
Participant information sheet
For this form, you will need to cover:
- Investigator details – your name, email address, work address and work contact telephone number (not your personal mobile or home phone number) and the same details for the responsible investigator (usually your supervisor)
- Purpose of the research – why are you doing the research?
- Who is doing the research and why – detail the research as part of a project/your PhD research.
- Exclusion criteria
- What participants will be asked to do
- Whether participants will need to attend sessions and where these will be located
- How long participation will take
- Disadvantages and risks of participating
- Possible benefits of participating
- Anything participants need to bring with them
- Information on data protection, right to withdraw, confidentiality, how their data will be used and what to do if participants are unhappy with how the research was conducted
Informed consent form
Informed consent forms usually follow a template, but you may want to add optional/additional elements such as:

Risk assessment
The template for your organisation/university may differ to the one used by mine (Loughborough University), but it is likely that you will need to think of all the activities that your research will involve, the activity’s associated risks, who might be harmed, measures to control the risk and then rate the likelihood and severity of the risk to calculate a ‘risk rating’.
Examples of things you need to think about for risk assessment:
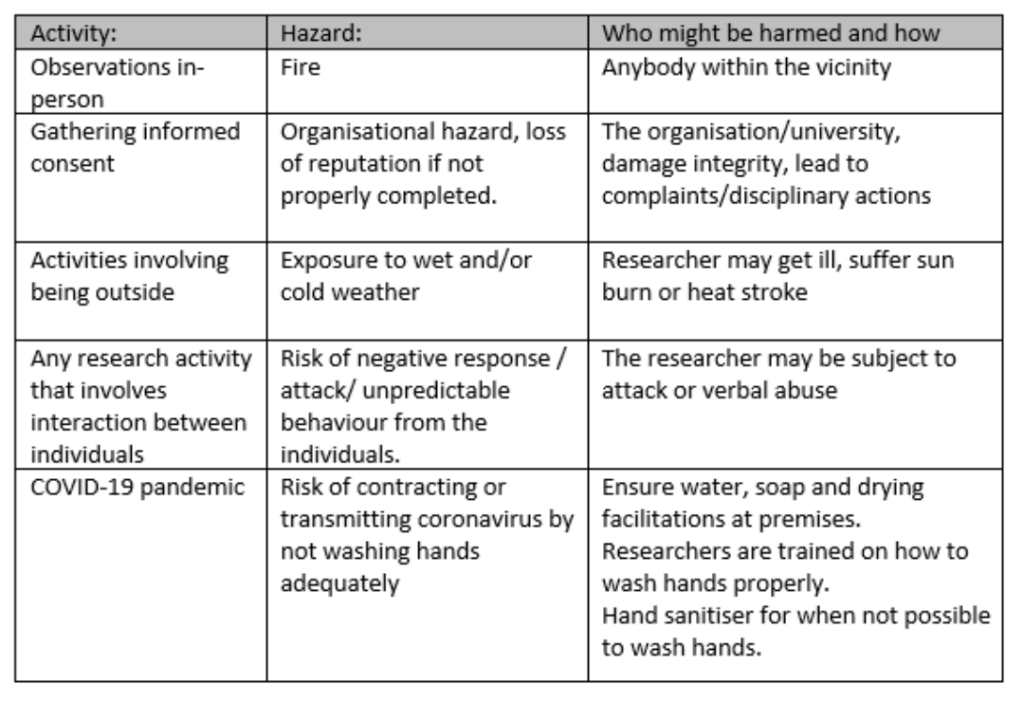
Sample questions/topic guide
If you are conducting research involving online surveys, interviews or focus groups you will need to provide an idea of the questions/topics you will discuss for them. For this, you need to think about what demographic information you need to collect – e.g., age, gender identity, race, occupation. There may also be demographic information specific to dementia which you need to collect. For example, asking if the participant knows anyone living with dementia, is or has been a carer for someone with dementia, or whether they are diagnosed with dementia themselves. You need to think about how personal these questions are.
In terms of surveys, you want to reduce drop out of participants part way through the survey as much as possible. Techniques for doing this include limiting ‘free response’ boxes and replacing them with Likert scale questions with an optional follow-up for participants to explain their answer. For example:
- To what extent do you agree with the statement “X, Y, Z”? *Required
- Strongly agree
- Agree
- Disagree
- Strongly disagree
- Please explain your choice: *Optional
- Free response _____
 It is also important to only ask questions on things you absolutely need to know as part of the research aims so participants are not overloaded with surveys which are unnecessarily long. Try to make the survey visually clear and easy to follow – but this part can come after the ethics submission. For the ethics submission, it is only needed that you give the committee enough idea of what you will be doing, and tweaks can be made afterwards. It is also important to show that you have incorporated signposting into the survey design (e.g., a link to support groups, organisations, and charities).
It is also important to only ask questions on things you absolutely need to know as part of the research aims so participants are not overloaded with surveys which are unnecessarily long. Try to make the survey visually clear and easy to follow – but this part can come after the ethics submission. For the ethics submission, it is only needed that you give the committee enough idea of what you will be doing, and tweaks can be made afterwards. It is also important to show that you have incorporated signposting into the survey design (e.g., a link to support groups, organisations, and charities).
For focus groups, it is important to include a topic guide (i.e., questions and prompts around the topic(s) you plan to discuss). You will also need to include information about how you will introduce the focus group to the participants (e.g., housekeeping information about fire alarm procedures, rules such as ‘listen to others’ and ‘don’t speak over each other’, repeat how long the focus group is expected to take, remind participants you are interested in their opinions/beliefs, recap of their ethical rights – right to withdraw, confidentiality, right to refuse to answer or leave the room at any time, gain permission to record and detail when you will take a break). Remember that signposting will also be needed at the end of the focus group, this can be achieved in the follow-up email to participants so that online links are easier to access than if a paper copy were given.
Details of research document
In this document you may want to include extra details you feel are relevant to the proposed research which will allow the ethics committee more insight into your plans and show you have thought through the research. These details include:
- Rationale for the research (aims and objectives)
- Lay or non-specialist summary of study background
- Study method (description of study and methodology)
- Participants (participant details, participant selection and recruitment, storing data safely)
- Participant safety (vulnerable participants)
- Investigator experience (your prior experience with the research methods, population etc.)
Ethics and dementia research
The advice I have described so far has been general, but I would now like to consider what extra things we may need to take into account when conducting research with people living with dementia. Some of the points I will describe are related to in-person research/interviews/focus groups:
- Fatigue to participants – How much are you asking participants to do? Are you scheduling in enough breaks? Do you need to ask all the questions, or will fewer do? Will you provide refreshments? Are you reminding participants that they can leave the room whenever they like?
- Consider anything you could offer participants which may be useful to them – in the case of my research, I plan to run a blog writing workshop for participants and provide a platform for them to publish on as a way of thanking them for their participation. Consider what may be useful for participants in your research.
- Consider whether you are exposing participants to harm through your research – i.e., will you be discussing potentially upsetting topics? If so, you will need to show you have thought about how you can help participants access any help they may need by directing them to relevant charities and organisations such as Alzheimer’s Society or Carers UK. You will also need to let participants know they can decline to answer any question and can leave the room at any time.
- For interview or focus group research, or indeed any research which is quite involved, it is a good idea to hold an initial meeting so that participants living with dementia can get to know one another, the researcher and ask any questions. This situation also allows the researcher to assess whether a participant looks comfortable in participating and they can be asked if anything is worrying them or if they would like to withdraw.
Dementia and informed consent
This is a large area of concern in dementia research, but if you are aware of relevant legislation, and have checks in place, you will be fine. Legislation that you need to be aware of is The Mental Capacity Act (2005), which is good to read up on. In terms of consent, I have found the process model of consent useful. This involves monitoring and reviewing consent throughout the duration of the research project, asking family members or informal carers if this is something the participant living with dementia is likely to want to participate in as well as asking for consent from the participant living with dementia, and looking out for signs of them no longer wanting to be part of the research (these signs could be non-verbal and behavioural cues as well as verbal language). In terms of my own research, I am only allowing participants to self-select to take part. This is so that individuals can elect to take part (recruited from the online survey and through other means if necessary, such as the DEEP network and social media). This does however exclude people with more advanced dementia who may be less able to communicate.
- Find out all the relevant information and templates before beginning your ethics submission – especially dates for when the committee meets to discuss submissions!
- Include enough detail so that the committee gets a real idea of how your research would work
- Consider how people living with dementia will need to be supported, what you can offer them as well as what they can offer you
- Consider how you will combat possible upset caused to your participants from participating
- Read up on ethics and relevant literature in the field
- Ethics and dementia journal papers
- Papers on inclusive research with people living with dementia
- Information for NHS ethics guidance

Felicity Slocombe
Author
Felicity Slocombe is a first year PhD Student from Loughborough University. Felicity’s research focuses on identity and dementia and how identity can be managed interactionally – how we can help support identity of people living with dementia through our conversations. Driven by a family connection to dementia, and writing each month on a range of topics from her work, and that of her wider group ACTInG (Applied Cognition Technology and Interaction Group), and sharing news from her training and events.

 Print This Post
Print This Post






