Western Blotting has a bit of a reputation in the academic circles – for being, well, temperamental, unforgiving and witchcraft at its finest. Us researchers that have been lucky enough to run one – or many – of these bad boys, don’t have the best memories of this technique. We often joke about making sacrifices to the Western Gods or that the Western will only work if the tank faces north-east. But – jokes aside – in this blog I will describe the basis of what a Western is, what it can tell us scientifically and how I have used it in my own work in the lab.
Western blotting or immunoblotting, is a laboratory technique which is used to detect a specific protein – or proteins – within the complex mix derived from cells or tissues. Westerns can help us understand how much – or how little – a protein is expressed, or in other words how abundant it is, determine if proteins are being up- or downregulated, detect cellular location of proteins, as well as giving us an understanding of the size of the protein and if any of these measures might change for example, in disease, or after a treatment.
But before we even get near the Western Blot tank, the first step is deciding what sample we are going to run. Some tissue samples, like the brain, include many different proteins that are all expressed at different levels. Some proteins have lower expression and thus may be trickier to detect. In my work, I was particularly interested in proteins that make up the blood vessels of the brain and to understand how the composition of these proteins may change in small vessel disease. Blood vessel and basement membrane proteins are notoriously difficult to detect, so, to give myself the best chance of seeing these proteins I developed a protocol to enriched my brain samples for blood-vessel specific proteins. My new brain sample then included many more vessels per volume than if I compared it back to my original ‘whole’ brain sample.
So now that we have our sample, we need to decide what type of Western we want to run. This is determined by what proteins we want to detect and how we can access them with antibodies. In a denaturing Western – it sounds painful, I promise it isn’t – we boil our sample in a special buffer, known as anionic detergent sodium dodecyl sulfate (SDS) to break the structure of the protein. We do this, because antibodies typically only recognise a small portion of the protein of interest i.e the epitope and the epitope may reside within the 3-D structure of the protein which would be inaccessible unless we unfold or denature it. For some proteins this is not necessary, in which case we wouldn’t denature our sample at all, which occurs in a native Western.
After our sample preparation we are ready to embark on the first step of Western Blotting – pause for drum roll – gel electrophoresis. To put it simply, this technique separates the sample complex protein either by charge (native Western) or by weight (denaturing Western). If we denatured our sample, as described above, the SDS buffer allows all proteins to become negatively charged, as they attach to the SDS anions. The negative charge is proportionate to the length of the proteins, therefore migration though the length of the gel is determined by molecular weight. In other words, heavy proteins will run slowly and stay at the top of the gel and small proteins will run quickly and end up at the bottom of the gel. This step is achieved by loading our sample into a well of a gel in a special Western blotting tank, then when an electric current is applied to the gel, the negatively charged proteins will migrate towards the positive charge – at the bottom of the gel – and separate by size. The end result – hopefully – is a gel with lots of sequential lines on it.
Sounds pretty straight forward, right? But lots can go wrong at this stage. If the current or buffer isn’t right this can lead to smiling or flowing lines. And depending on how wrong this is, it can lead to all different shapes. Twitter is flooded by researchers sharing their Westerns gone wrong, and so far, the best shape I have ever seen, must be this bull, courtesy of Dr Sophie Quick1.
Once the proteins have run and separated, they are transferred out of the gel to the surface of a membrane. This transfer of proteins can happen in a couple of different ways and will be determined by what size proteins you are interested in. All transfer types follow the same principle of the sandwich – which ironically happens around lunch time! – where the gel and membrane are sandwiched together with blotting paper and sponges. An electric current is then applied causing the proteins to migrate out of the gel and attach to the membrane. Needless to say, that the order of the sandwich is crucial, if not assembled correctly the proteins will either transfer onto the blotting paper or simply float away into the transfer buffer. Bubbles aren’t your friends either, they can cover your proteins, making it impossible to quantify your protein expression at the very end.
Yes, remember when I said that Western blotting is not forgiving? I wasn’t joking.
Once the transfer has taken place, we can take a breather and set the tank aside. Our protein-full membrane is blocked to prevent non-specific binding from loose proteins that may have occurred during the transfer and then probed with specific antibodies that bind to the target proteins of interest. This binding is then detected with a chromogen or a fluorescent tag. And finally, the membrane is imaged to reveal the expression – or lack – of the protein or proteins of interest. This data can then be quantified – where the intensity of each band can be measured and normalised to a control protein, thus measuring protein expression for each sample.
In my own work I have used Westerns for a couple of different reasons. Remember my blood-vessel enriched samples that I mentioned previously? With these samples I was able to quantify expression of four key basement membrane proteins where I was comparing across control mice and mice with a collagen IV mutation – the same human mutation that leads to a form of genetic small vessel disease. I was able to measure the expression of these proteins and observed different levels of them in control and disease mice and that this expression pattern changed across brain regions. I have also used Western blots as a confirmatory tool for another type of brain enrichment, and then sent these human and mouse samples for proteomics analysis2 – i.e the master technique for large-scale protein study.
Away from the lab, Western blotting has also been used in a clinical setting, to aid in the diagnosis of infectious diseases including HIV, Lyme disease, hepatitis C, syphilis as well as some autoimmune disorders. Although, it’s gradually been replaced by the arrival of new, more sensitive and specific diagnostic assays that have been developed for some diseases. Thus, Western blotting remains a predominately research rather than clinical tool3.
Despite the challenges and yes, the frustrations that Western blotting can bring, it is still an invaluable tool in research to study and detect proteins and also has applications in a diagnostic setting.
And just remember to always make sure to turn your Western tank to face north-east!
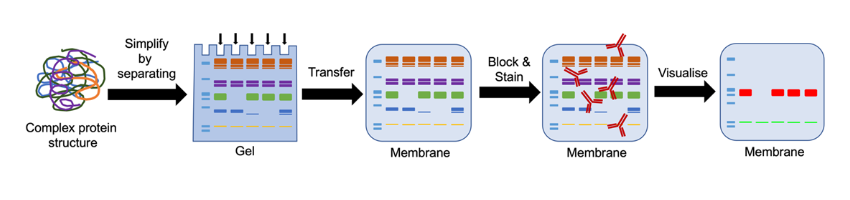
Schematic of a Western blot. Firstly, you start with a complex protein structure, say a piece of brain tissue. You then try and simplify that complex structure by separating the proteins contained within it on a gel via gel electrophoresis. You then transfer the proteins from the gel onto a membrane which can be blocked, stained with antibodies and tags to detect and subsequently visualise your proteins of interest.
References
- https://twitter.com/sophiefquick/status/1640788438591873037?s=46&t=HEq-83lR4DeCoVE4z8BIKQ
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8392779/
- https://www.aacc.org/cln/articles/2015/october/the-past-present-and-future-of-western-blotting-in-the-clinical-laboratory

Dr Gaia Brezzo
Author
Dr Gaia Brezzo is a Research Fellow based within the UK Dementia Research Institute at The University of Edinburgh. Gaia’s research focuses on understanding how immune alterations triggered by stroke shape chronic maladaptive neuroimmune responses that lead to post-stroke cognitive decline and vascular dementia. Raised in Italy, Gaia came to the UK to complete her undergraduate degree, and thankfully, stuck around. Gaia writes about her work and career challenges, when not biking her way up and down hills in Edinburgh.

 Print This Post
Print This Post





